Published on the 13th June 2017 by ANSTO Staff
ANSTO has contributed to research led by a group at Oxford University that demonstrated the viability of using a single-step double element-hydrogen bond activation process to produce a metal dihydride compound.
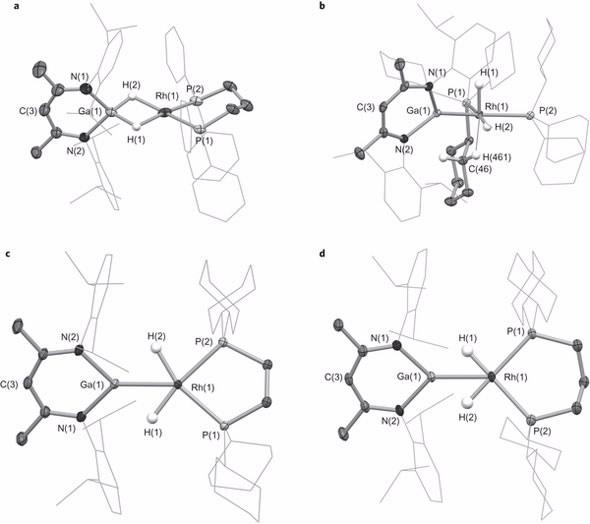
The Aldridge Group, led by Prof Simon Aldridge at the Inorganic Chemistry Laboratory, University of Oxford, successfully isolated and characterised a series of compounds representing the reaction intermediates along a bond activation pathway by varying the attached phosphine ligands.
Understanding how bonds are activated is fundamental to numerous chemical transformations, including key catalytic transformations in synthetic, medicinal and materials science, and to dehydrogenation processes in the petrochemical industry.
The study, which was published in Nature Chemistry, defines a new oxidative activation pathway and promises systematic control over the extent of the bond activation processes leading to the formation of transition metal complexes, for example featuring carbenes.
The paper reports significant findings from the doctoral projects of Joe Abdalla and Alexa Caise, both members of the Aldridge Group. Based in Oxford, this group specializes in the synthesis of novel Main Group and organometallic complexes, and investigation of their underlying electronic structure and fundamental patterns of reactivity.
 |
| Key bond lengths and angles for intermediate species: a, 2-dppp. b, 2-PCy3. c, 2-dcype. d, 2-dcypp |
Neutron diffraction, at the Australian Centre for Neutron Scattering was undertaken by Dr Alison Edwards, a specialist in structural chemistry and chemical crystallography. This led to the elucidation of the molecular structures of the cationic components of the intermediate species.
“Single-crystal Laue diffraction on the Koala instrument, which revealed the location of the metal bound hydrogen atoms of the cations, enabled us to establish the nature and extent of transfer of the hydrides,“ said Edwards.
The synthesis of the new compounds, crystal growth, X-ray diffraction studies and theoretical modelling were all undertaken at the University of Oxford.
Theoretical modelling was used to probe the electronic structure and provided insight into the nature of the chemical reactivity of the intermediate species.
The publication marks the 50th paper to be produced using measurements on the KOALA Laue diffractometer, a leading instrument of its type in the world.
DOI: http://dx.doi.org/10.1038/nchem.2792