
Published on the 17th September 2019 by ANSTO Staff
Experiments at ANSTO’s Australian Centre for Neutron Scattering and the Australian Synchrotron have revealed the growth mechanism behind an unusual twin crystal that forms conjoined “wings” in the most commonly prescribed antihypertensive and diuretic drug, hydrochlorothiazide (HCT).
Dr Alison Edwards, a co-author on the paper published in Angewandte Chemie the ‘butterfly’ crystals had clear left and right-handed domains that shared a common plane.
Although molecular chirality (when a molecule has a mirror image that is not superimposable) in pharmaceutical compounds was well known, the distinctive structure of symmetric conjoined crystals was unusual.

Twin crystals comprise domains of opposite chirality
“This research, which was led by Prof Mark Spackman at the University of Western Australia and his post-doc Dr Sajesh Thomas (now a Marie Curie Fellow at Aarhus University in Denmark), is valuable because of what it teaches us about crystallisation processes and spontaneous resolution that might occur or be encouraged to occur” said Edwards.
Control of crystallisation processes in an industrial setting is important, such as in the manufacture of drugs.
“We know there are some molecules used in drugs, in which one hand of the molecule is therapeutic and the other is not or may even be harmful.
“There are examples with drugs for epilepsy, in which one hand prevents convulsions and the other causes them.
“Control of the growth of the desired form of a medication is critical.
“There have been tragic cases where drugs were brought to market in an approved form as a mixture of its right and left-hand forms – the best known is Thalidomide as described in Scientific Reports in 2018. The area remains of considerable ongoing importance in pharmacy. The issues controlling the mode of action of this and other drugs also remain an important area of research – control of chirality is fundamental in this regard and our paper provides insight into this.
“Another classic example is limonene, which has a side that tastes of lemons while the other tastes of oranges. The difference in flavour comes from the chirality of the molecule.
“Understanding how crystals start, or nucleate, and grow is extremely useful information.
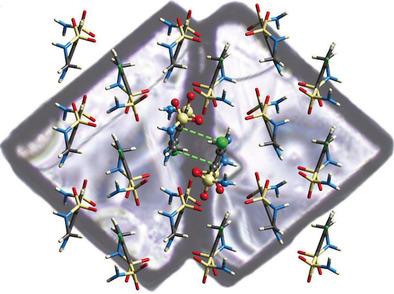
“In HCT, the structure begins from a common face with left and right-handed domains in a specific orientation.
“We have known that the molecule did this for a very long time without understanding the mechanism at work,” said Edwards.
There is only one other previously known case of a molecule with a mixture of domains, known as enantiomers, exhibiting a boundary between two physically separable domains.
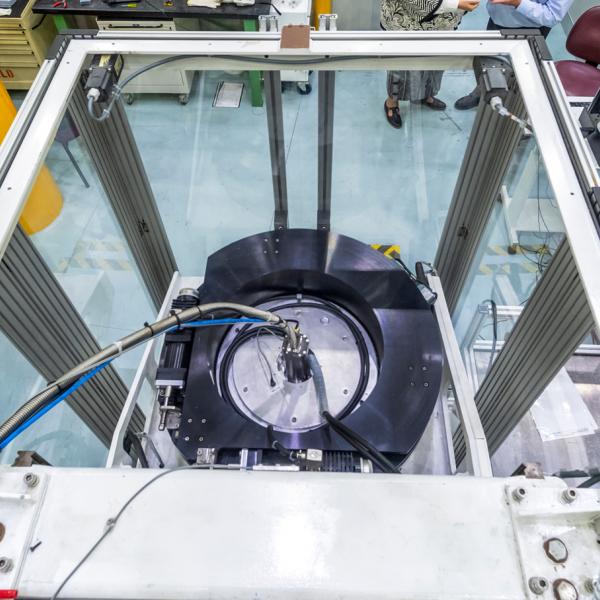
“Our neutron diffraction study on the Koala instrument was undertaken to determine the position of hydrogen atoms in the molecule and how they interact with each other.”
“Because neutrons interact with the nucleus of hydrogen atoms, we get real intermolecular distances.”

Dr Alison Edwards and Dr Ross Pilz confer at the Koala Laue Diffractometer
Dr Ross Piltz, also a co-author, assisted with data refinements.
The investigators also used micro X-ray diffraction data at the Australian Synchrotron HCT to confirm the crystal structure of the intact twin wings and the separated wings of the twin crystal.
Circular dichroism spectroscopy also helped differentiate the enantiomorphic twin crystals from other types of twin crystals.
The authors noted that despite its common use, the subtle conformational chirality and persistent appearance of symmetric co-joined twin crystals of HCT (Form 1) were not known.
For more information