
Published on the 3rd November 2023 by ANSTO Staff
Key Points
-
Research provides insights into the formation of structures in rechargeable lithium ion batteries that will help prevent catastrophic failure
-
Surface area and interfacial distances of deposited lithium change in complex ways depending on the history of the battery's use
-
SANS and USANS techniques provided a precise and less complicated way to analyse the structure of deposited lithium
In recent research published in Advanced Energy Materials, a team of ANSTO scientists, led by Prof Vanessa Peterson, used neutron scattering techniques to understand the formation of harmful lithium structures in rechargeable lithium ion batteries (LIBs).
Despite being found in most portable electronics and electric vehicles, the energy capacity of LIBs falls short of that required by many next-generation technologies. Although replacing the common electrodes in these batteries with pure lithium metal can help the battery store much more energy, lithium microstructures that form at the lithium surface can create short-circuits, and lead to catastrophic battery failure.
‘It is important to understand how and why these detrimental lithium structures form in order to prevent them from forming, and ultimately enable us to use these types of higher energy batteries’ said Prof Peterson.
It was known that there were different types of structures, ‘whiskers,’ ‘moss,’ and ‘dendrites’ in dismantled batteries. Whiskers resemble tiny needles, moss looks like a porous layer, and dendrites are long, thin structures, and it is these ‘pointy’ structures that cause the most trouble.
“We used small-angle and ultra-small-angle neutron scattering (SANS and USANS) techniques with our Quokka and Kookaburra instruments at the Australian Centre for Neutron Scattering to study these complex lithium structures,” said Prof Peterson.
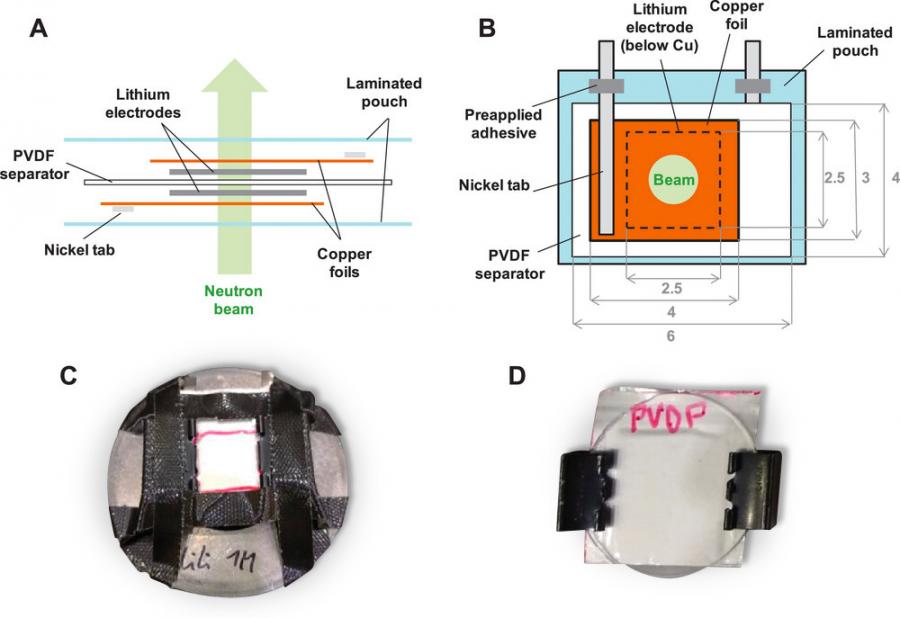
She explained that these methods are insightful because they provide information about the size and shape of the lithium structures inside a battery without taking it apart. Because a battery contains many components, SANS and USANS data were collected from these different parts in order to isolate the information coming from the lithium only in the data.
“This information helped us design a symmetrical pouch cell that was ideal for studying the changes in the lithium that gets deposited inside it.”
The investigators used SANS and USANS to provide a precise and less complicated way to analyse the structure of deposited lithium compared to other methods like X-ray imaging, microscopy, or gas adsorption.
The study also revealed that SANS and USANS were sensitive to the development of interfaces between the lithium and the electrolyte due to lithium deposition.
Prof Peterson used mathematical models to quantify the surface area and the average distance between these interfaces based on the data obtained from the measurements.
“We observed that the surface area and interfacial distances of deposited lithium change in complex ways depending on the history of the battery's use,” said Prof Peterson
“This research opens the door for future investigations to explore how factors like the amount of electric current, charging time, and the cyclic process of lithium deposition and dissolution impact the surface area and the distances between interfaces within deposited lithium.
“Understanding these factors is crucial for addressing the challenges associated with lithium dendrite growth in LMB technology,” she said.
ANSTO contributors include first author energy materials specialist Dr Christophe Didier (who completed a contract at ANSTO), Prof Elliot Gilbert and A.Prof Jitendra Mata.
ANSTO’s specialist capabilities and expertise in the analysis of materials used in energy applications continue to provide important insights into batteries.