Published on the 7th November 2023 by ANSTO Staff
A large Australian team led by Monash University has devised a new approach to killing antibiotic-resistant bacteria using lipid nanoparticles that target specific layers on the surface of the bacterial cell.
Research published in the journal Small demonstrated that antibacterial lipids can be successfully used in combination with nanocarrier lipids to form nanoparticles that kill gram-negative bacteria.
This approach expands the possibilities of methods for the delivery of antibacterial lipids in combination with established treatments to treat bacterial infections.
The research team was led by Prof Jian Li, Prof Anton Peleg, and A/Prof Hsin-Hui Shen of Monash University.
Instrument scientist and co-author Dr Anton Le Brun contributed to the research with measurements on the neutron reflectometer Platypus and analysis of the data.
“Neutron reflectometry is a useful tool for understanding the structure of cell membranes at the nanometre length scale,” explained Dr Le Brun
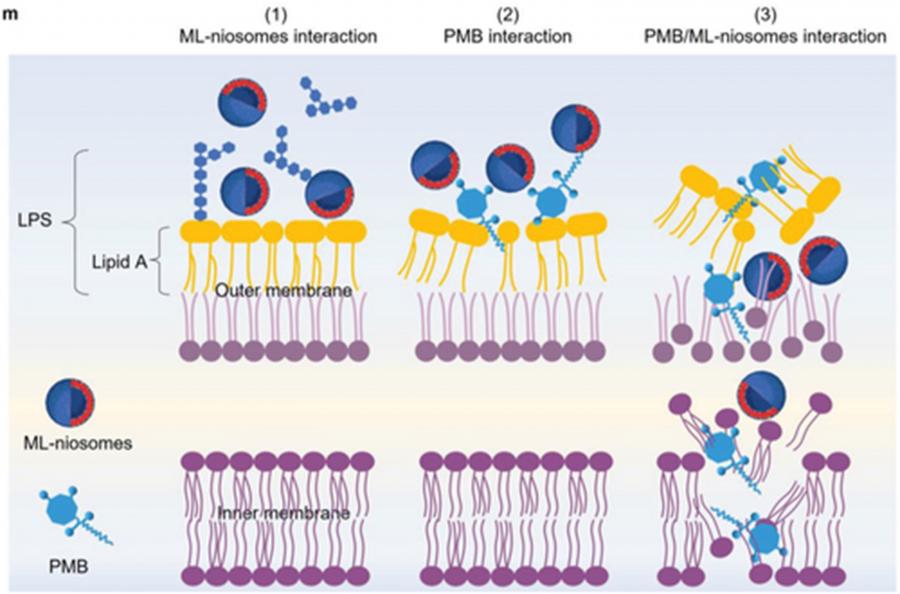
The Platypus instrument at the Australian Centre for Neutron Scattering was used to elucidate the mechanism at work in a combined ML-niosome/polymyxin B treatment at the molecular level.
Niosomes are vesicles with special properties that are used to deliver drugs.
Polymyxin B is an antibiotic of last resort for treating infections from gram-negative bacteria. However, some bacteria are starting to show signs of resistance even to this antibiotic.
By making artificial membranes that mimic the properties of the gram-negative bacterial cell surface, the team discovered that the ML-niosomes target the outer layer of the outer membrane, which is mainly composed of polysaccharides.
The binding of the ML-niosomes to the surface of the outer membrane exposes the membrane. It gives polymyxin B better access to attack and breakdown the protective outer membrane and then the inner membrane —ultimately killing the bacterial cell.
The new complex was effective against to a wide selection of hypervirulent strains of K. pneumoniae, A. baumannii, and P. aeruginosa, including multidrug-resistant pathogens.
Future work will investigate how this is achieved in detail at the molecular level and why the combination with polymyxin B is more effective.
Other steps will expand the study to test against other pathogens where resistance to established treatments is proving problematic.
Contributing organisations included CSIRO and the Doherty Institute.




